The Indiana Myeloma Registry
Attaya Suvannasankha, MD
Primary Investigator
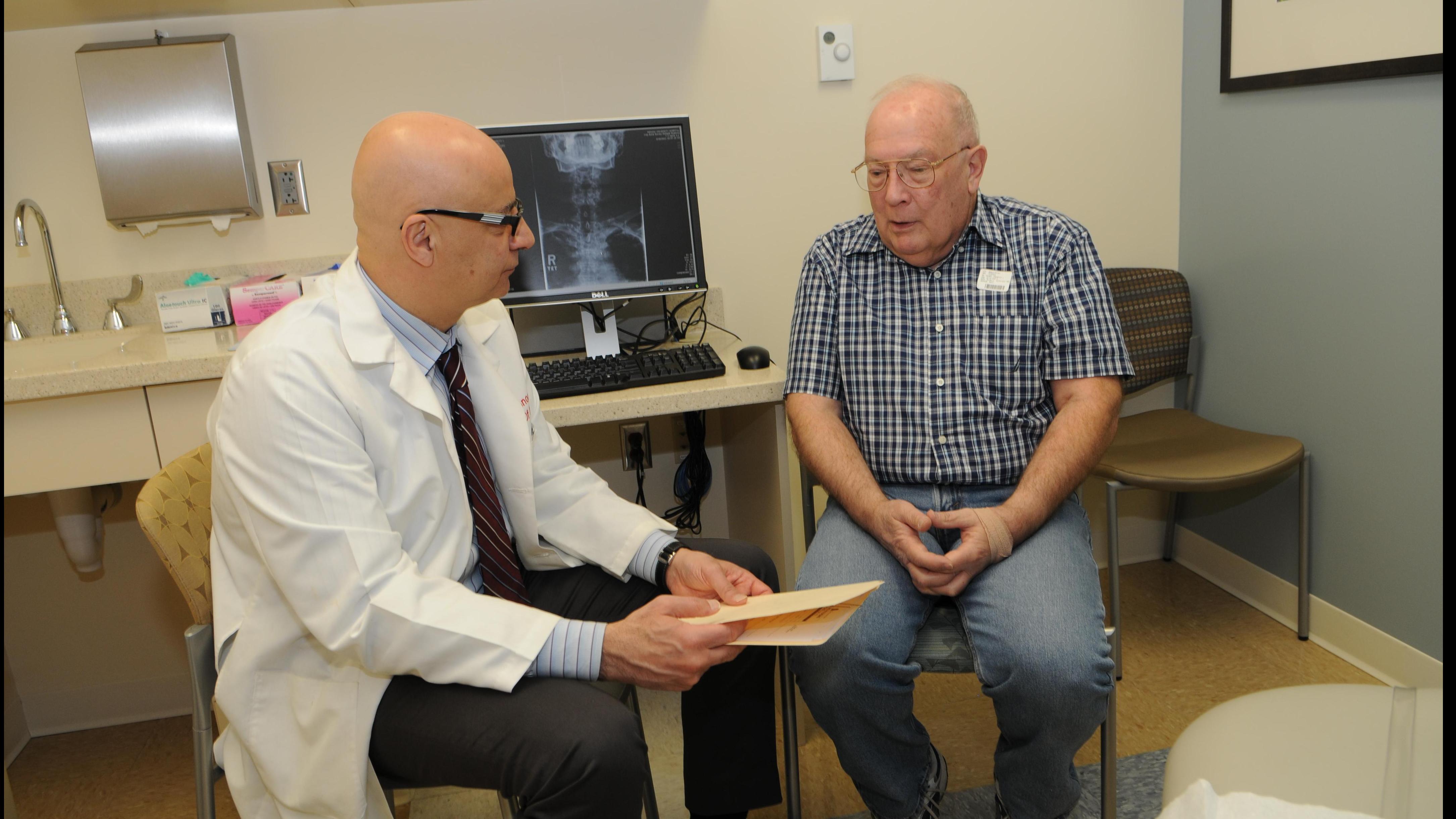
Brief description of study

What is cancer?
![]()
![]()
Multiple Myeloma is cancer of the plasma cells
![]()
![]()
![]()
![]()
![]()
![]()
![]()
There's still a lot we don't know about Multiple Myeloma. That's why research is so important.
- Who is most likely to get Multiple Myeloma?
- What treatments work for which people?
- What causes Multiple Myeloma?
Study Parts
SALIVA SAMPLE | HEALTH HISTORY QUESTIONNAIRE | QUALITY OF LIFE QUESTIONNAIRE | APPROVAL TO LINK TO YOUR MEDICAL RECORD |
|
| ||
We'll mail you a kit! | One time: online or by phone | Every 3-12 months: online or by phone | So we can follow how your treatments go without you doing extra work! |
Study Eligibility
- You are a male or female patient age 18 or older at the time of informed consent
- You have been diagnosed with or are suspected to have one of the following:
- Monoclonal Gammopathy of Undetermined Significance (MGUS)
- Smoldering Multiple Myeloma
- Multiple Myeloma
- Bony or Soft Tissue Plasmacytoma
- Primary Amyloidosis
- Plasma Cell Leukemia
- Other Plasma Cell Dyscrasias (including but not limited to light chain deposition disease, monoclonal gammopathy of renal significance, POEMS syndrome)
Detailed description of study
Frequently Asked Questions:
No, we can mail a saliva sample kit directly to you and all other study activities can be done online or by telephone.
2. How is the data secured?
Efforts will be made to keep your personal information confidential. We cannot guarantee absolute confidentiality. Your personal information may be disclosed if required by law. No information and databases in which results may be stored which could identify you will be shared in publications about this study.
3. Is DNA shared with anyone else?
Information or specimens collected from you for this research may be shared with other researchers in the future. If shared, your information will only be usedhe continued study of multiple myeloma and related diseases (monoclonal gammopathy of undetermined significance, smoldering multipleyeloma, amyloidosis, and plasmacytoma). If this happens, we will not share your personal identifiers. We do not plan to ask your consent before we share this information. Use of your information by other researchers will require review and approval from the Indiana Myeloma Registry Steering Committee and if appropriate, approval by the Institutional Review Board. The Institutional Review Board reviews research proposals to make sure your rights and welfare are protected.
4. Will the study change my treatment?
No,directly. You will still work with your own doctors for your treatment plan. The study may help us find better treatments for Multiple Myeloma patients that you could benefit from in the future. Theudy may also help you find clinical trials that you might be eligible.
5. Does the study cost anything?
No, it's completely free for you.
6. Will I get results of my DNA analysis?
No, not currently. We are hoping to do this in the future.
7. Will my employer or my insurance company see my study information?
No. And there is a law called GINA that makes it illegal for employers or insurance companies to discriminate against you based on your DNA.
Eligibility of study
You may be eligible for this study if you meet the following criteria:
- Conditions: POEMS Syndrome,Plasma Cell Leukemia,Plasma Cell Dyscrasias,Amyloidosis,Monoclonal Gammopathy of Undeterminded Significance,MGUS,Plasmacytoma,Multiple Myeloma,mileloma,cancer,
-
Age: 18 years - 100 years
-
Gender: All
- Has been diagnosed with or is suspected to have one of the following:
- Monoclonal Gammopathy of Undetermined Significance (MGUS)
- Smoldering Multiple Myeloma
- Multiple Myeloma
- Bony or Soft Tissue Plasmacytoma
- Primary Amyloidosis
- Plasma Cell Leukemia
- Other Plasma Cell Dyscrasias (including but not limited to light chain deposition disease, monoclonal gammopathy of renal significance, POEMS syndrome)