Vape Study: Help us Understand Nicotine Dependence with Brain Stimulation
Joshua Brown
Primary Investigator
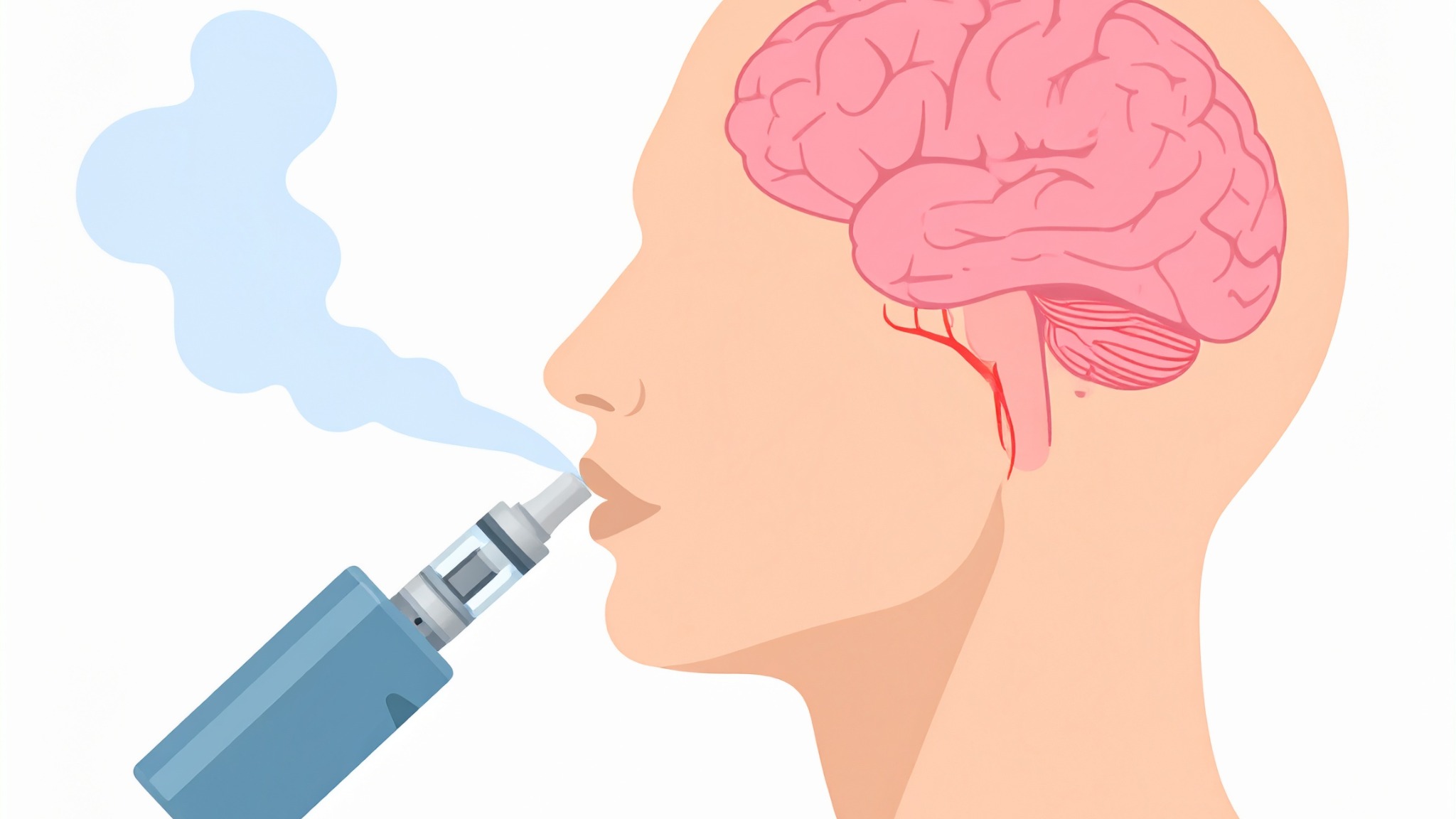
Brief description of study
Do you use nicotine-based vapes?
Our study is testing the effects of brain stimulation on nicotine use and craving. Temporal interference (TI) delivers very mild electrical currents to the scalp that reach deeper brain regions linked to addiction, without surgery.
You may qualify to be in this study if you:
- Are between the ages of 21 and 50
- Have at least a 6th grade education and are English-speaking
- Vape at least 15 mg of nicotine per day (~1-2 cigarettes/day)
- Are not currently in treatment for nicotine use
Detailed description of study
If you are eligible and choose to participate, you will attend one in-person study visit lasting 2-3 hours either at Indiana University in Bloomington, IN or the IU School of Medicine in Indianapolis, IN.
- You will be asked to abstain from nicotine use for 8 hours prior to your in-lab appointment. *You will also be asked to provide a urine sample at the beginning of your session.
- During the study visit, you will receive mild brain stimulation using a gentle electrical current or sham (fake) stimulation while vaping nicotine. Participants are randomly assigned to one of three conditions: stimulation targeting the nucleus accumbens (linked to motivation and craving), stimulation targeting the anterior insula (linked to awareness of urges), or a sham condition with no active stimulation.
- You will be asked to rate your cravings during the session and to complete brief follow-up surveys on your smartphone for seven days, as well as one additional survey two weeks later.
- You will receive $40/hour for the lab session, plus $5/day for 7 days of EMA reporting with a $10 completion bonus, and an additional $10 for completing the 2-week follow-up survey. Total compensation is approximately $80–$175 for completion of study requirements.
*The urine sample will be used for two purposes: (1) to screen for recent drug use and (2) for female participants of childbearing potential, to conduct a urine pregnancy test. These tests are required for safety reasons, to ensure that participation in the study does not pose additional health risks. The results of these tests will not be shared with anyone outside the research team. If your urine sample tests positive for drug use or pregnancy, you may not be eligible to participate in the study.
Eligibility of study
You may be eligible for this study if you meet the following criteria:
- Conditions: Nicotine dependency, Vape, Vaping, Healthy
-
Age: 21 years - 50 years
-
Gender: All
- Adults aged 21–50
- Nicotine dependent; vape ≥15 mg nicotine per day
- Smartphone with internet access
- At least 6th grade education
- Able to read/speak English
- Are currently not seeking treatment for nicotine use
- History of seizures/seizure disorders (including family history)
- Severe or hard-to-treat migraine headaches
- Suicide attempts or thoughts of suicide in the past month
- Heart rhythm problems (such as irregular heartbeat, long QT, pacemaker, or other heart disease)
- Hypertension with systolic BP >150 mmHg
- Brain conditions or injuries that affect structure or blood flow (such as tumors, stroke, multiple sclerosis, or brain bleeding)
- Serious mental health conditions (such as schizophrenia, bipolar disorder, psychosis, or dementia)
- Head injuries that caused loss of consciousness or bleeding (such as skull fractures or subdural hematomas)
- Current use of opioids, cocaine, or methamphetamine
- Severe cannabis (marijuana) use disorder
- Alcohol use disorder or a history of alcohol withdrawal
- Metal implants in the head or under the scalp (except dental fillings or implants)
- Current use of stop-smoking treatments or medications that affect reward or craving
- Pregnancy