Loading...
Found 768 All Conditions trials
A listing of All Conditions medical research trials actively recruiting patient volunteers. Search for closest city to find more detailed information on a research study in your area.
C
Craig Sewell
This study investigates a condition called systemic mastocytosis, where the body produces too many mast cells due to a change in the KIT gene. This can be advanced (AdvSM) or non-advanced (NonAdvSM). The investigational medication offered in this study aims to target the KIT gene change to reduce abnormal mast …
O
Onyedika Ilonze
This study investigates the use of an ECG-based artificial intelligence (AI) device to assess the risk of undiagnosed pulmonary hypertension (PH) in patients with interstitial lung disease (ILD). The purpose of this study is to determine if the device can increase the rate of new PH diagnoses compared to the …
K
Kathy Miller, MD
This study investigates how safe and effective two investigational agents, an imaging agent and a therapeutic agent, are for patients with advanced breast cancer. The imaging agent, called 68Ga-R11228, is used to locate specific tumor cells in the body. The therapeutic agent, 177Lu-R11228, is used for treatment. Both agents are …
E
Emma Tillman
This study investigates what happens when people with Cystic Fibrosis (PWCF) who have experienced mental health or liver-related issues with a previous medication start taking an investigational medication. Cystic Fibrosis is a condition that affects the lungs and digestive system, caused by a defective gene that leads to thick, sticky …
J
Joel Corvera, MD
This study investigates the safety and effectiveness of using an investigational device for treating Type A aortic dissections. Aortic dissection is a serious condition where the inner layer of the aorta tears, causing blood to flow between the layers of the wall of the aorta. This study is specifically for …
M
Myda Khalid
This study investigates the safety and effects of an investigational medication in people with autoimmune kidney diseases. These conditions include IgA Nephropathy (IgAN), Primary Membranous Nephropathy (pMN), and Minimal Change Disease/Focal Segmental Glomerulosclerosis (MCD/FSGS). Autoimmune kidney diseases occur when the body's defense system attacks the kidneys, causing damage. This study …
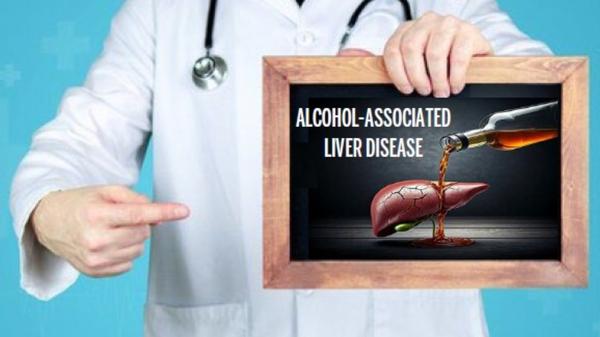
R
Raj Vuppalanchi, MD
Do you have liver damage because of excessive alcohol use? Join our study!Researchers at the Indiana University School of Medicine are looking for adult volunteers to take part in a study looking at the link between heavy drinking and liver disease.Your participation may help researchers to gain knowledge that may …
C
Caroline Rouse, MD
This is a prospective, observational study which is evaluating the obstetrical, neonatal, and cardiovascular outcomes of 1000 pregnant people with known heart disease to define how best to structure cardio-obstetrics care to optimize outcomes.THIS STUDY IS ENROLLING BY INVITATION ONLY - This study is enrolling participants from specified community and …
D
Devon Mackay
This study investigates how certain surgical positions, such as Trendelenburg positioning (TP) and pneumoperitoneum (PP), might affect the optic nerve and vision in people with idiopathic intracranial hypertension (IIH). Idiopathic intracranial hypertension is a condition where the pressure around the brain increases, which can lead to optic nerve swelling known …
R
Rohan Maniar
This study investigates the effects of adding an investigational medication, cemiplimab, to the usual treatment of docetaxel and ramucirumab for patients with stage IV or recurrent non-small cell lung cancer. This type of lung cancer is advanced and may have returned after previous treatment. Cemiplimab is a monoclonal antibody that …